ARENSIA is a German operator of professional research clinics, specialized to conducting exploratory clinical trials encompassing patients across a wide range of disease areas.
ARENSIA's research-dedicated infrastructure enables a remarkable time reduction in patient recruitment compared to typical clinical trial sites burdened by overcrowding, staff shortages, population competition, and lengthy contract negotiations. The shortened timeframe and the streamlined setup of focusing efforts on a select few ARENSIA Clinics (instead of the traditional large number of sites) result in an overall budget reduction of more than 50% for a typical Phase IB/IIA PROOF OF CONCEPT trial.
Dedicated to conduct most complex Phase IB/IIA/PROOF OF CONCEPT protocols in patients
Full time medics specialized in numerous disease areas
Strategic locations in Europe, North America and North Africa enable access to immense pools of patients in all therapeutic areas.
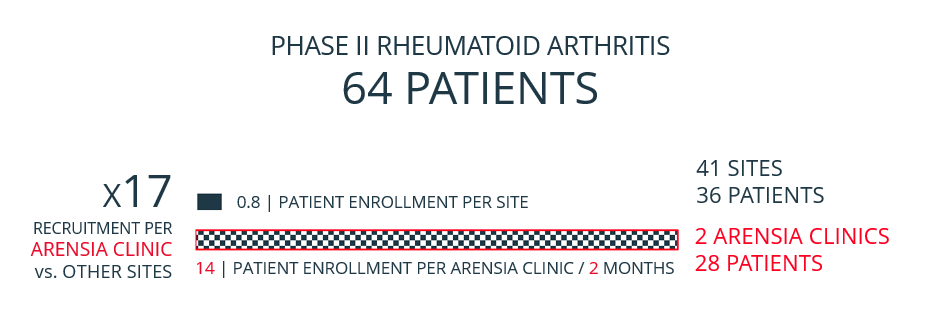
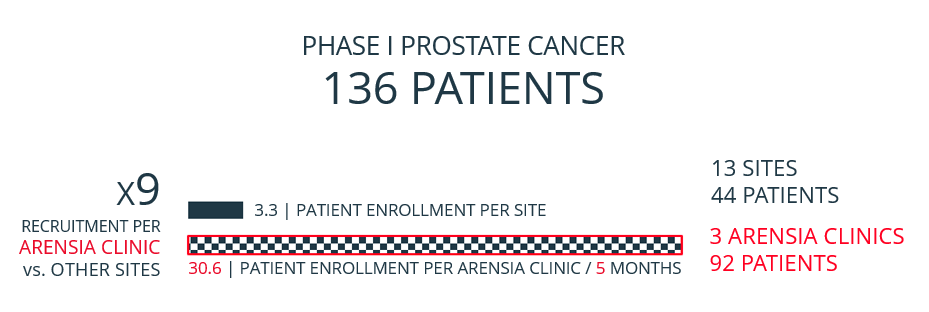
ARENSIA consistently oversees protocols involving frequent PK sampling, sophisticated biomarker collection (including imaging and biopsies), as well as patient hospitalization and/or ambulatory visits.
ARENSIA's research clinics comprises 10-25 surveilance beds per each clinic following a unified standard SOP system with on-site:

Dedicated to the performance of Phase I / II / PROOF OF CONCEPT protocols involving patients

A dedicated study coordinator at each location is assigned to manage the entire cycle of the project in ARENSIA clinics

We offer 10+ years of expertise in the preparation and submission of dossiers to HA & EC at each ARENSIA clinic

We advise Sponsors on scientific and operational aspects of their exploratory trials
ARENSIA'S SPEED AND QUALITY PROVIDE A STRATEGIC VALUE ACROSS THE ENTIRE LIFECYCLE OF A PROJECT!
The world's leading pharmaceutical, biotechnology, and global clinical research organizations rely on ARENSIA as their favoured site solution for Phase IB/IIA/PROOF OF CONCEPT trials that involve patient participation.
See what our Sponsors and CRO partners said about us.
In regards to patient enrollment, ARENSIA fulfilled their recruitment commitment and went on to enroll more patients than initially assigned. The ARENSIA Team demonstrated very high medical proficiency, trustworthiness and business sophistication.
We have very much appreciated the opportunity to work with ARENSIA and together further pioneer science. Not only did you help us enroll the study in record time, you have worked diligently to help us with a successful cohort lock. We look forward to continuing our partnership.
We are extremely happy and impressed with our partnership with ARENSIA. I already said a few times in our calls with the Sponsor and my team that I have never seen such a pace and successful collaboration in my 21 years in the industry.
We encourage Sponsors with compounds approaching Phase I or II for which patients are required, to consult us as early as possible in the concept phase of the protocols. Our clinics are glad to accommodate in person visits or live virtual tours.
On a non-binding and free of charge basis, we offer scientific and operational feedback as well as feasibility of timelines, enrollment pace, and budgetary considerations.